-
About Us +
Company Profile Enterprise Digital and Intelligent Corporate Culture Qualification & Honor
- News +
-
Products & Solutions +
CDMO Services for Innovative Drugs Generic Drug API and Intermediate Services Product Center
- R&D+
Chiral Drugs Synthesis and Analysis Technology Crystal Form & Salt Form Screening Technology Biological Enzyme Engineering Technology Continuous Flow Engineering Technology Photochemical Technology
- Quality & Regulatory+
Quality Policy Operation of the Quality System Regulatory Affairs Quality Audit and Registration Verification
- ESG+
EHS Policy Three-Standard Integrated Certification Safety Management Occupational Health Energy Conservation and Environmental Protection IP Protection System Business Ethics Management System
- Recruitment +
- Contact Us +
Results
- About Us + Company Profile Enterprise Digital and Intelligent Corporate Culture Qualification & Honor
- News and Information + Company News Information Notice
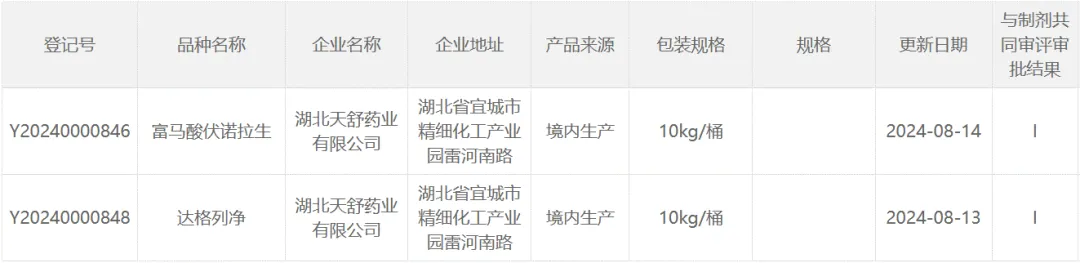
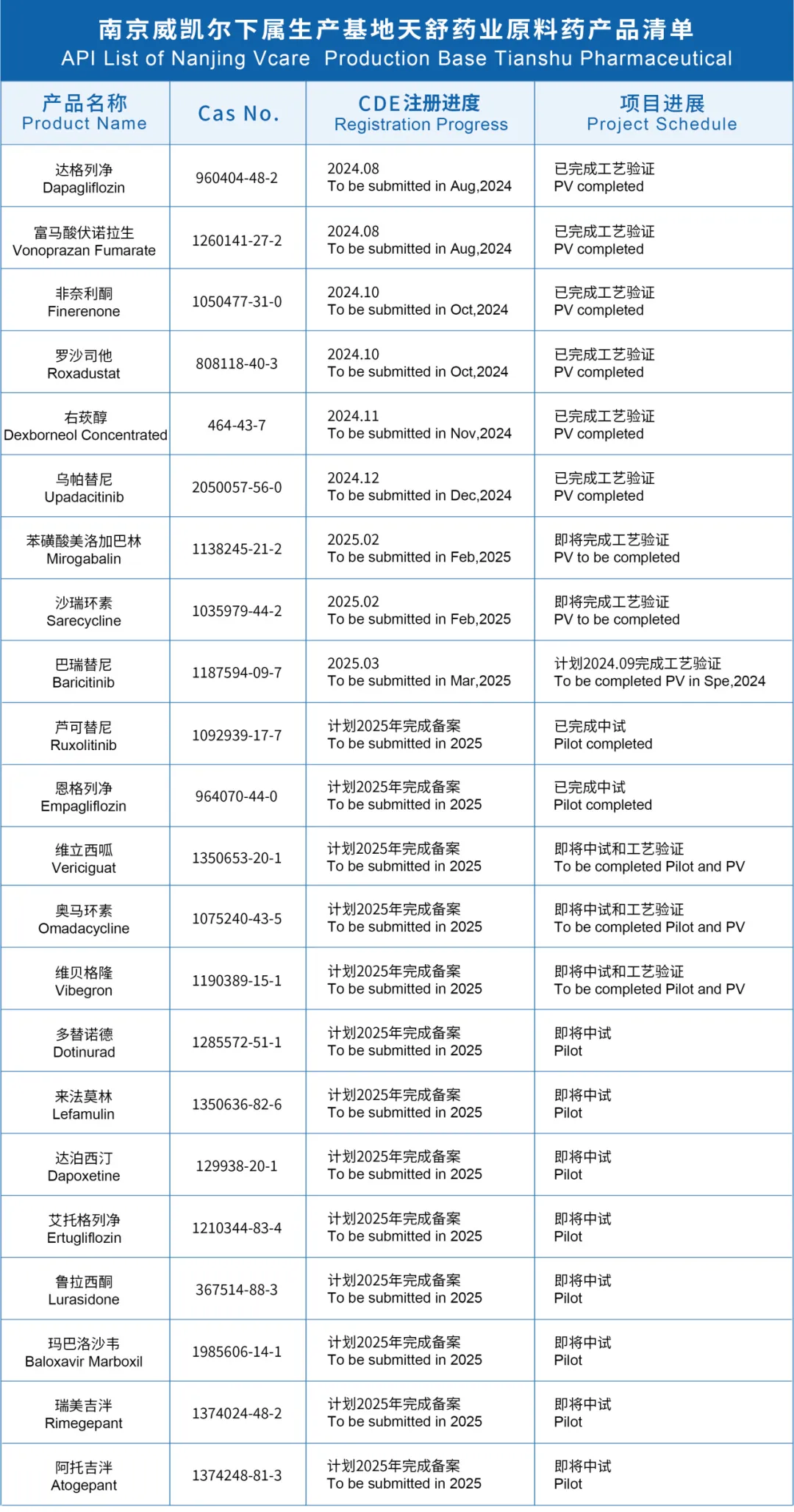
- 产品与解决方案 我们的服务 Product Center ─ Characteristic API ─ Advanced Intermediate ─ CDMO ─ Impurity
- R&D and Innovation R&D and Innovation
- Quality System and Regulatory Affairs Quality System and Regulatory Affairs
- ESG ESG
- Talent Recruitment + Talent Development Recruitment Position
- Contact Us + Online Message Contact Information